Heat energy and change of phase
Heat energy and change of phase
Comfort air conditioning systems add heat to air in the winter and remove heat from air in the summer. However, the heat exchanges that result in sensible temperature change are just one part of the total air conditioning function. Other processes, including the removal (summer) or addition (winter) of moisture the air (humidity control); the provision of a suitable amount of “fresh” outside air (ventilation), and the filtration and distribution of conditioned air are all very important aspects of the total air conditioning problem. We will be concerned with heat exchange in two forms:
(1) the kind that causes a temperature change, which involves what we call sensible heat; and
(2) the kind that causes a substance to undergo a change of state, which involves what we call latent heat.
Heat-exchange processes constitute the very core of air conditioning theory and practice. A thorough understanding of them is essential before we can begin to work at any level in the air conditioning industry. We will explain how heat is measured and how heat-exchange processes can cause a change of state.
Heat energy
When a substance undergoes an increase in temperature we say that heat has been added to it, and if its temperature falls we say that heat has been removed. These changes in temperature reflect changes in the total internal energy of the atoms and molecules of the substance. Heat gains and losses do not always result in temperature changes, however. Under certain conditions, a gain or loss of internal energy causes the substance to change its state rather than causing a change in temperature.
In air conditioning we are directly concerned with heat energy and with the methods of computing heat losses and gains. The molecular energies of different substances are not equal even at the same temperature, and it is therefore necessary to select some substance as a standard and express the energies of all other substances in terms of that standard. Water is the standard substance for heat-energy measurements, and unit heat quantity is defined as the amount of heat required to raise the temperature of one kilogram of water one degree Celsius. This quantity of heat is called the kilo-calorie (kcal).
1 kcal amount of heat required to raise the temperature of 1 kg of water 1 °C
Conversely, as 1 lb water cools through a temperature range of 1 °F, 1 Btu of heat is given off. The dimensions of the Btu are lb°F.
1 Btu amount of heat required to raise the temperature of 1 lb water 1°F
Heat energy properties of substances other than water are expressed by means of the concept of specific heat capacity (c). The specific heat capacity of a substance is the number of kilocalories that must be added to or removed from one kilogram of a substance to change its temperature by one Celsius degree. The specific heat capacity of water expressed in metric units is 1.00 kcal/kg°C.
In the USCS system it is the Btu that must be added to or removed from one pound of it to change its temperature by one Fahrenheit degree. The specific heat capacity of water is therefore 1.00 Btu/lb°F.
All ordinary substances have specific heat capacities less than unity (see Table). In other words, water has the highest specific heat capacity of all common substances. The numerical values of specific heat capacities are the same in both the metric system and USCS. Why?
Since the dimensions of the kcal are kg°C and those of the Btu are lb°F (and since 0.454 kg is the weight equivalent of 1 lb of mass and 1 °C =1.8 °F), it follows that
252 cal = 0.252 kcal = 1 Btu
and
1 kcal = 3.97 Btu
Heat Calculations
The quantity of heat a substance will absorb as it warms up, or give off as it cools, is directly related to its weight (or mass, in the metric system) the change of temperature, and the specific heat capacity of the substance.
This relationship is known as the basic heat equation (metric),
H = mc(t2 – t1)
where H is in kcal, m in kg, c is specific heat capacity and (t2 – t1) in °C
and in USCS,
H = wc(t2 – t1)
where H is in Btu, w is in pounds, c is specific heat capacity, and (t2 – t1) is the temperature change, in °F and in USCS
Mechanical Equivalent of Heat
Count Rumford’s early experiments on the nature of heat were in themselves indicative that a probable relationship existed between mechanical work and heat energy. It remained for a later investigator, James Prescott Joule (1818-1889), to determine the exact relationship between heat energy and mechanical energy. Joule found that 778 ft-lb of mechanical work was the equivalent of 1 Btu of heat energy. The relation was determined by observing the exact temperature rise of a known quantity of water as a measured amount of mechanical work was done on the water by rotating paddle blades.
Joule’s experiments, verified by many subsequent workers, evaluated the mechanical equivalent of heat as 778 ft-lb/Btu. This value is an important physical constant and can be expressed as
J = W/H.
The equation is also written
W = JH
J = W/H = 778 (ft.lb)/BTU
in which W is mechanical work in foot-pounds, H is heat in Btu, and J is Joule’s constant, also known as the mechanical equivalent of heat. In order to distinguish the symbol J, for joules, and J, the symbol Joule’s constant, the former always appears in bold type and type and the latter in italics.
J is for joules (the metric unit of energy)
J is for joule’s constant, the mechanical equivalent of heat
In SI units, Joule’s constant is usually expressed as the ratio
J= W(kilojoules)/H(kilocalories)
or
J = 4.187 kJ/Kcal
To convert USCS units of heat to metric units, recall that l Btu = 0.252 kcal. From this,
1 Btu = 1.055 kilojoule (kg)
The branch of physics which concerns itself with the relationships between heat and mechanical work is known as thermodynamics. The mechanical equivalent of heat concept merely means that:
- 778 ft-lb of mechanical energy will produce 1 Blu of heat energy when expended on nor useful work (e.g., friction).
- 1 Btu of heat energy, if it were used by a heat engine which was 100 Percent efficient, would produce 778 it-lb of mechanical energy!
Since, in air conditioning problems, we are frequently concerned with heat gains and losses expressed in Btu and also with power input to driving motors or engines in kilowatts or horsepower, the importance of Joule’s constant can readily be appreciated.
Metric units and constants can be substituted for the USCS units in the paragraphs numbered (1) and (2) above, and the meaning will be unchanged.
Heat Capacity Expressed In Energy Units
It will be noted in the specific capacity table above has a column giving specific heat capacities in kilojoules per kilograrn-Celsius degree, (kJ/kg.°C). The metric system expresses heat energy in joules and kilojoules and the rate at which heat energy is supplied in joules per second, or watts (or kilowatts).
The relationship between kW and kcal/sec is:
1 kcal/sec = 4.186 kW
or
1 kW = 0.239 kcal/sec
Specific heat capacity in kJ/kg.°C is obtained by multiplying the values of c given in kcal/kg.°C by J, the mechanical equivalent of heat, that is, by 4.186 kg/kcal
The Three States of Matter
At ordinary temperatures and pressures, different substances commonly exist in three different states. There are the solids, such as metals, wood, and minerals, the liquids, such as water, alcohol, and gasoline, and the gases, such as oxygen, carbon dioxide, and ammonia. These substances are well known to most of us, and their solid, liquid, or gaseous state under normal temperature and pressure conditions is merely a matter of observation. Engineers and technicians must, however, consider the behavior of substances not only at ordinary temperature and pressure conditions but throughout whatever range of conditions may be encountered in the air conditioning problem.
We are accustomed to thinking of water as a liquid but know that it becomes a solid (ice) at temperatures below 0°C (32°F), and becomes a gas or vapor (steam) at temperatures above 100°C (212°F), when pressure is atmospheric. Similarly, lead is normally a solid but becomes a liquid at 326°C (620°F) and will vaporise if heated to 1620°C (2950°F). Carbon dioxide is normally a gas but will liquefy at -60°C (-76°F) and will become a solid (dry ice) at temperatures below -79°C (-110°F).
Pressure, temperature, and change of state
These few examples should serve to illustrate the point that the particular state which a substance happens to be in at the moment depends entirely upon the conditions of temperature and pressure then obtaining.
It is helpful to recall once again the molecular theory for assistance in understanding this concept. For example, consider water in the liquid state at room temperature and atmospheric pressure. Its molecules are in random motion, and molecular collisions are frequent. Molecules are far enough apart, however, to permit the fluidity which we associate with liquids. If the temperature is increased to 100°C (212°F), holding pressure constant at 1 atm, the water will boil and steam will be formed. This is a change of state from liquid to gas.
Steam, which is water in the gaseous or vapor state, has a molecular behavior markedly different from that of liquid water. Its molecules are much farther apart, molecular velocities are much greater, and it possesses an even greater degree of fluidity. It is also highly compressible, while liquid water is very nearly incompressible. Steam possesses the properties of a true gas.
If on the other hand, the original water sample is subjected a temperature less than 0°C (32°F), with pressure constant, ice will soon begin to form. Ice has the molecular characteristics common to solids; molecules are relatively close together, their motion is restricted to quasi-vibratory paths, molecular energies are lower, and fluidity and compressibility are no longer present to any appreciable degree.
Water has been used as the example in discussing these change-of-state processes merely because it is such a common substance and because its changes of state at atmospheric pressure occur at temperatures common to everyday experience. Scores of different substances undergo similar changes of state, but at different temperature and pressure (P-T) conditions.
In order to illustrate in diagram form the change-of-state relations described above, let us investigate the effect on 1 lb of water of varying its heat content while holding the pressure constant at 1 atm.
The Temperature-Heat (T-H) Curve for Water
Molecules are known to exhibit forces of mutual attraction similar to those which exist between finite bodies such as the stars, sun, and planets. Since these forces are evidently overcome in producing the molecular separations required to convert a solid to a liquid and a liquid to a gas, there must be certain energy relationships for different substances to bring about these transitions from one state to another. When sufficient heat energy is added to a liquid, it “boils” and vapor is given off. When enough heat energy is taken away from a liquid, it “freezes” and becomes a solid. Using water again as a familiar example, energy-state-temperature relationships will be diagramed for 1 lb of water from 0 to 212°F and beyond, at a constant pressure of 1 atm.
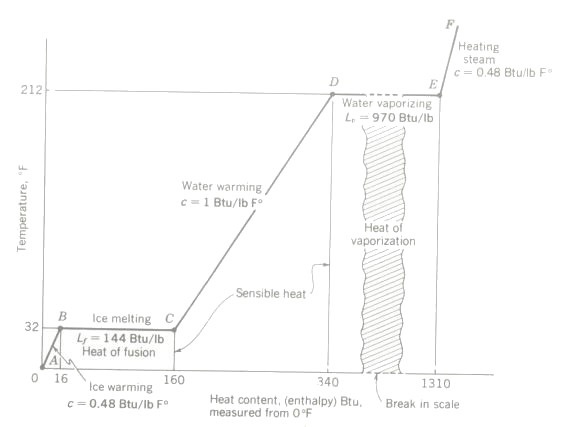
Referring to the temperature heat diagram above starting point is 1 lb of ice at condition point A (0°F) in the diagram. Temperatures are plotted along the vertical axis and heat content in Btu along the horizontal axis, measured from an arbitrary zero at 0°F.
As heat is slowly added to the ice, the temperature is found to rise 1°F for each 0.5 Btu added. (This indicates a specific heat capacity (c) of 0.5 for ice.) When 16 Btu have been added, the temperature of the ice will have risen to 32°F. This process of warming the ice is represented by the segment AB of the T-H diagram. Note that as heat is added, temperature rises. Since a rise in temperature can be detected by our senses, we call this sensible heat.
Sensible heat may be defined as heat which, when supplied to or removed from a substance, produces a sensible eject on the substance, that is, a change of temperature which is measurable by a thermometer.
Referring again to the diagram, 1 lb of water is now at condition point B, still solid ice, at a temperature of 32°F. Its heat content, measured from 0°F, is 16 Btu.
If we now add more heat to the ice, it is noted that no further temperature change occurs but the ice starts to melt. If heat is added slowly until all the ice has melted to water, it will be found that 144 Btu will be required to bring about the change of state from 1 lb of (solid) ice at 32°F to 1 lb of (liquid) water at the same temperature, 32°F. The sample is now at condition point C on the diagram, total heat content 160 Btu, measured from 0°F.
Since the addition of heat during the melting process BC does not result in a temperature change, it is not sensible heat. We cannot measure its effects by a thermometer. It is in a sense hidden from us, and so it is called latent heat.
Latent heat is defined as heat which, when supplied to or removed from a substance, produces a change of state without any change it temperature.
The latent heat of fusion Lf is the heat which must be added to one pound of a solid already at its melting-point temperature to change its state to liquid at the same temperature. The latent heat of fusion is also the heat which if removed, will cause one pound of a liquid substance to change to a solid, still at the same temperature.
Lf (water) = 144 Btu/lb
Returning again to the experiment, let more heat be added to the pound of (liquid) water. Immediately the temperature starts rising again, so this is sensible heat. As the water warms, it will be noted that for each Btu added, the temperature rises 1 F . (This is, of course, the basis for the definition of the Btu.) When the temperature reaches 212°F, we shall have added another 180 Btu and have arrived at condition point D on the diagram. Total heat (from 0°F) is now 340 Btu.
Continuing to add heat, it is noted that no further temperature increase occurs but boiling begins and liquid water becomes vapor. Another change of state is taking place along the DE segment of the diagram. It will require 970 Btu of heat to vaporize completely the pound of water and bring it to condition point E of the diagram, still at 212°F, with a total heal of 1310 Btu. Note that this total heat is made up of sensible heat and latent heat.
The latent heal of vaporization Lv is the heat which must be added to a pound of a liquid (already at its boiling-point temperature) to change its state to vapor at the same temperature. The latent heat of vaporization is also the heat which if removed, will cause one pound of a vapor to condense to a liquid.
Lv (water) = 970 Btu/lb at 212°F or 100°C
Further heating will result in increasing the temperature of the vapor (steam) and EF is therefore a sensible-heat process. This process requires 0.48 Btu for each °F temperature rise of the steam, so 0.48 for heating steam at atmospheric pressure.
Lf = 80 kcal/kg = 334 kJ/kg
Lv = 540 kcal/kg = 2256 kJ/kg
for water at 1 atm pressure.
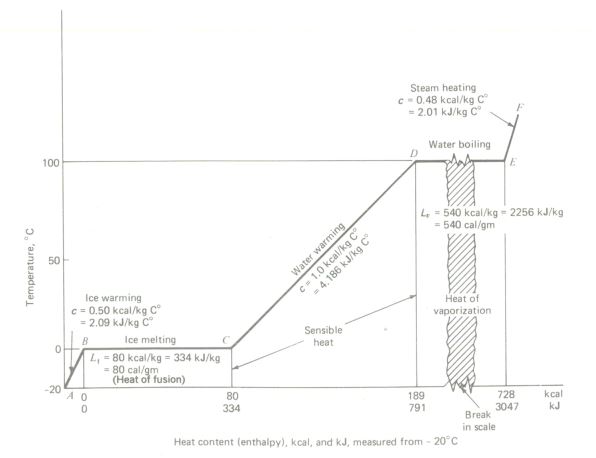
Temperature-heat (T-H) diagram for 1 kg of pure water at atmospheric pressure, with all values in the metric system.
Air Conditioning and Heat-Content Measurements
Heat-content measurements are of extreme importance in air conditioning work. The analysis just completed reveals that total heal content is the sum of sensible heal and latent heal. The term enthalpy is used for total heat content.
Enthalpy (total heat content) = sensible heal + latent heat
In air conditioning processes, heat is continually being added to or taken away from air, water, refrigerants, people, foods, and the various solid materials of the air-conditioned building.
Boiling as a Change-of-State Process
In air conditioning we are concerned with boiling in two different ways-water boiling in boilers to supply stream for steam heating system, and refrigerant boiling (evaporating) in the cooling coils or chilling apparatus of refrigeration systems.
The average person without technical or scientific training might say that boiling occurs when a liquid is heated to a temperature hot enough to cause vigorous bubbling. Although this is a satisfactory description of the boiling process as observed by the non-technical person, it does not suffice for air conditioning technology, because it will not explain the change-of-state processes on which the science of air conditioning is based.
The Concept of Vapor Pressure
The kinetic-molecular theory of heat was discussed in a previous chapter, and along with it the concept of a Maxwellian distribution of molecular velocities
in a given substance was presented. Let us apply these kinetic-molecular ideas to what might actually be happening in a beaker of water as it rests on a laboratory table. A thermometer may indicate that the water temperature is 60°F, but this is merely an indication of the average velocity of its molecules. Some of its molecules at any given instant may have zero velocity and therefore possess very low energies associated with temperatures at or near absolute zero. Conversely, others may be traveling at prodigious speeds and possess energies ordinarily associated with the temperature of superheated steam. Collisions are frequent.
These moving molecules are at any instant darting in all directions, and some of them move upward and hit the surface layer of the liquid. Those of low and medium energies (velocities) do not escape through the surface layer (because of surface tension) but many high-energy molecules do escape and form a vapor layer above the liquid surface. This is what is happening as any liquid evaporates.
As these higher-energy molecules burst through the liquid surface, they create a pressure called vapor pressure, which is opposed by the pressure of the atmosphere pushing down on the liquid. Since only the molecules of higher velocity are successful in escaping the molecular attraction of the surface layer, simple mathematics tells us that the average velocity of the molecules remaining in the liquid is lowered. There has been a net decrease in the total internal energy. This checks with experience, for evaporation does lower the temperature of a liquid.
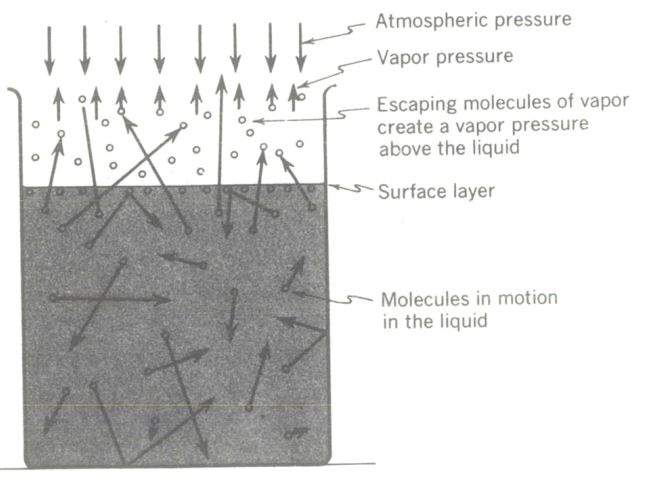
Molecular explanation of evaporation. The higher energy molecules can escape through the surface layer (surface open to the atmosphere), but lower energy molecules do not have enough energy to break the surface cohesion barrier.
Evaporation Is a Cooling Process
People are cooled by evaporation of perspiration from the skin and refrigeration is accomplished by evaporating the liquid refrigerant. As any given quantity of liquid evaporates without heal being supplied from an outside source, the liquid itself furnishes the heat energy needed to cause the change of state. Sensible heat on the liquid is converted to latent heat in the vapor leaving the liquid. As sensible heat is converted to latent heat, the temperature of the remaining liquid falls. If no heat is supplied from or surrendered to the outside, this is a constant-heat process. Such constant-heat processes are known as adiabatic, and we call this type of cooling adiabatic cooling. Evaporation is a cooling process.
Condensation, on the other hand, is a heating process. As molecules of vapor condense and become liquid, their latent heat of vaporization evidences itself again as sensible heat, indicated by a rising temperature. This heating effect of condensation is what causes the considerable rise in atmospheric temperature often noted as fog forms and as rain or snow begins to fall.
Saturated Vapor
If a cover is placed over the beaker, creating a confined space above the liquid, a saturated vapor will be produced. As the process of evaporation takes place, more and more molecules escape into the confined space and become vapor. They collide with other vapor molecules, and many fall back into the liquid. Eventually a condition of equilibrium will be reached in which the same number of molecules fall back to the liquid as escape from the liquid in a given time. The space above the water surface is then said to be saturated with vapor. The pressure of the vapor reaches a maximum as saturation is reached, and this maximum value is called the saturated vapor pressure. The presence of air or other gases does not affect the ultimate value of the vapor pressure itself. Evaporation will continue, regardless of the presence of other gases, until a partial pressure due to vapor, which is equal to the saturated vapor pressure of the liquid, is reached (this is known as Dalton’s law of partial pressures).

Molecular explanation of saturated vapor. When equilibrium occurs at the surface layer, the vapor pressure above the liquid is called the saturated vapor pressure (closed container).
Recalling again the kinetic theory of heat, reasoning tells us that if the water were hotter, it would have an increased percentage of high-velocity molecules, more of them would be successful in escaping from the surface layer, and consequently a higher vapor pressure would result. This reasoning is borne out by experiment-the higher the temperature of a liquid, the greater its saturated vapor Pressure.
It can be considered either as a saturated vapor curve for water or as a boiling Point curve. It is also known as a Pressure-temperature (P-T) curve. Any pressure-temperature combination to the left of and above the curve (such as condition point A) results in a liquid, any P-T combination plotting to the right and below the curve (point B) indicates the vapor phase, and the curve itself is the locus of all P-T points in equilibrium between the liquid and vapor phases.
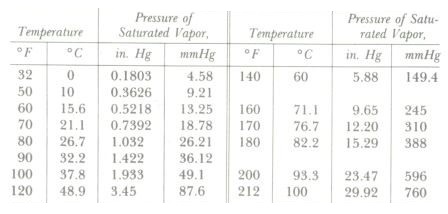
Saturated Vapor Pressure of Water at Various Temperatures
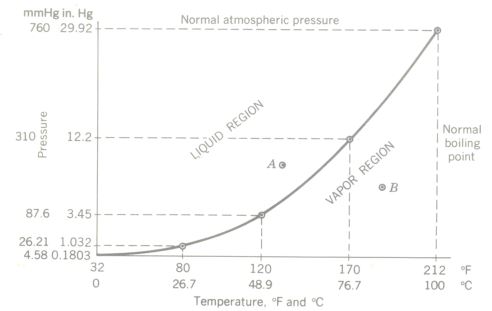
The vapor pressure curve for water. Such a curve is also known as a P-T curve or boiling point curve.
Vapor Pressure and the Boiling Point
Vigorous boiling consists of evaporation throughout the entire liquid, and the formation of bubbles would be impossible if the vapor pressure which forms them were not equal to (or slightly greater than) the external pressure acting on the bubbles. The external pressure is made up of the atmospheric pressure pushing down on the liquid surface plus the hydrostatic pressure of the liquid at the depth at which the bubble is forming. For a bubble forming at the surface, the vapor pressure must be at least equal to atmospheric pressure. We can now define boiling Point.
Boiling Point is that temperature al which the vapor pressure of the liquid becomes equal to (or slightly greater than) the external pressure on the liquid.
This definition opens up some very interesting lines of thought, which can best be pursued by discussing two simple experiments.
Boiling by "Cooling"
Fill a flask about half full of tap water. Select in advance a one-hole stopper which fits snugly in the Flask and in which a thermometer fits tightly. Boil the water in the unstoppered flask by the conventional method of heating it. The heat added raises the vapor pressure until it equals or slightly exceeds atmospheric pressure, and boiling begins. Check the temperature as being at or near 100°C (212°F) while vigorous boiling is in progress.
With the Flame removed and while working rapidly and carefully (!) insert the stopper and thermometer in the flask and immediately hold the entire flask under cold tap water for several seconds. Then invert it in a ring stand over a sink and note that the temperature is now significantly below 212°F, the so-called boiling point of water. Boiling has stopped by this time.
However, boiling can be made to commence again by cooling the flask! Pour cool tap water over the Flask, and brisk boiling begins again while the thermometer records a falling temperature! (The stopper must not allow air to leak into the flask). Those who has carefully thought through the kinetic molecular theory of heat and the vapor-pressure explanation of boiling will be able to explain this rather strange phenomenon of boiling by “cooling “
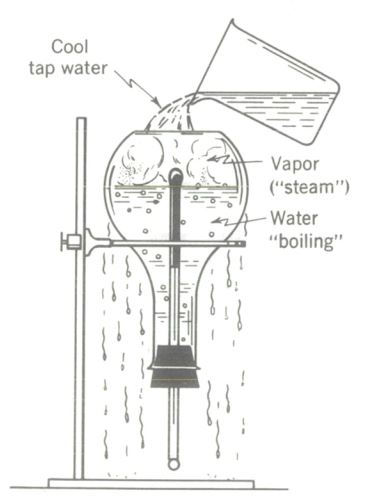
Boiling water by (apparently) cooling it.
Freezing by Boiling-the Triple Point
Prepare a vacuum pump, vacuum table, and bell jar SO that a high degree of vacuum can be attained. In a clean watch glass place a few tablespoons of tap water (temperature about 65°F) and support the watch glass over a small container of concentrated sulfuric acid (to help the pump absorb water vapor), all on the vacuum table. Put the bell jar over the apparatus, using vacuum wax for a good seal. There must be no leaks or the desired result will not occur. The vacuum table should be equipped with a good vacuum gauge, and a thermometer should be suspended from the bell jar so its bulb will be in the water. Start the pump, and take simultaneous pressure and temperature readings every 15 sec.
First, dissolved air comes out of the water in very small bubbles. Then, at a point (approximately) where P = 0.52 in. Hg and t = 60°F, boiling of the water begins. Boiling continues as both pressure and temperature drop. After a few minutes the temperature will have dropped to 32°F and pressure will be about 0.18 in. Hg. Bubbling will have slowed down or almost ceased. After another minute or so (watch closely for it) the water surface loses its sheen and the water suddenly freezes to ice. Ice formed, liquid was present, and vapor was being given off all at the same time! This condition point (P = 0. 18 in. Hg, I = 32°F, or 4.57 mmHg, 0°C, respectively) is known as the triple Point for water, and this demonstration is commonly known as the tripe-point experiment.
Water was boiled, not by heating, but by pulling a vacuum on it, and ice was frozen from boiling water. No Hame was required to furnish heat to boil the water. No refrigerant was used to freeze the water. Can you explain the processes involved in this experiment? If you have really thought through the kinetic molecular theory of heat, the Maxwellian distribution idea, the discussion of sensible and latent heat, the vapor-pressure explanation of boiling, and the real meaning of adiabatic processes, you can.
Water is used in the above examples for two reasons:
- It is the most common medium of heat exchange and is always present in the air to be conditioned, and
- the changes of state which we desire to show can be easily demonstrated for water with relatively simple equipment within a range of temperatures and pressures easily obtainable in the laboratory.
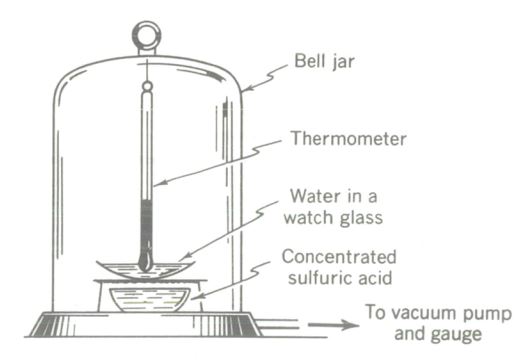
The triple-point experiment. At a very low pressure liquid water boils to a vapor as it simultaneously is freezing to a solid. (Note: Do not repeat this demonstration frequently, as the water vapor drawn into the vacuum pump may damage it.)
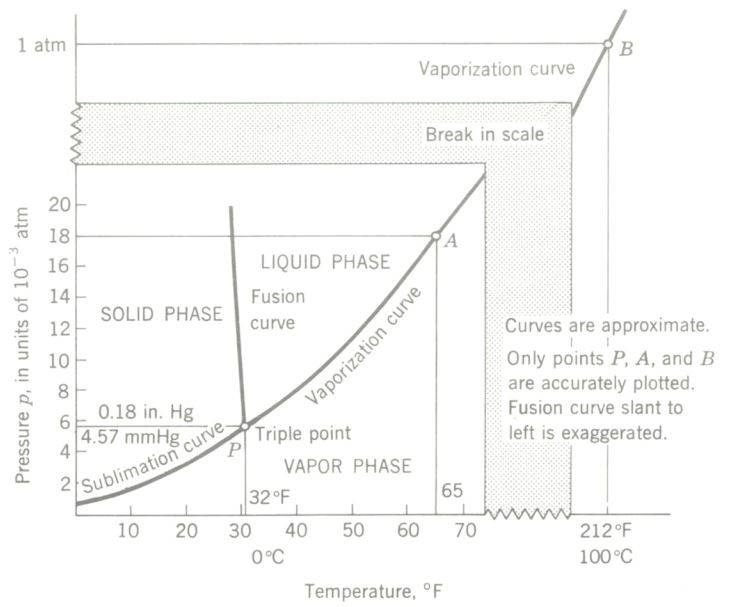
Phase diagram for water, showing the triple Point. At the triple point P, water exists in equilibrium among all three states-solid, liquid, and vapor.
Read more: Fan wall

