Relationship between Pressure and Temperature
Relationship between Pressure and Temperature
If you look at the water at a microscopic level, we see an enormous number of water droplets in it, and each of these droplets is itself made up of a vast number of water molecules.
To define a water molecule, let us say that it’s the smallest drop of water that can exist, and we’d need a mighty electron microscope to see one!
We can compare every water droplet to a balloon (the party type that keeps children happy – until they burst!)
When you blow into a balloon, you’ll notice that it expands. What happens is that the balloon’s membrane wall stretches due to the pressure of air that you blow into it.

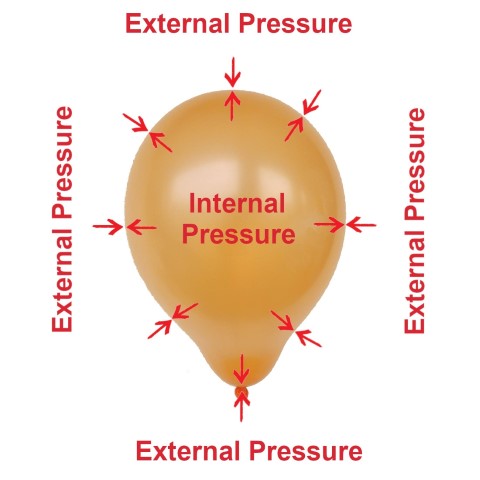
When you blow up a balloon, two pressures act upon the membrane.
- An internal pressure developed by the air that you’ve blown into it.
- Atmospheric pressure exists around us at all times.
And what happens if we increase the internal pressure a bit too much?
If you blow too hard, the internal pressure becomes too great, and the balloon explodes. Don’t you ever explode when someone puts you under too much pressure?
In the same way as the balloon we’ve just seen, every water droplet has external and internal pressures imposed on it.
When we put water in a saucepan, we observe that the water’s surface stays flat and horizontal. Also, the water stays in the pan and doesn’t generally splash into your face. Why?
Let’s consider this: we know that external and internal pressure exerts on every point on the surface of the liquid. In our example, the external pressure is due to atmospheric pressure.
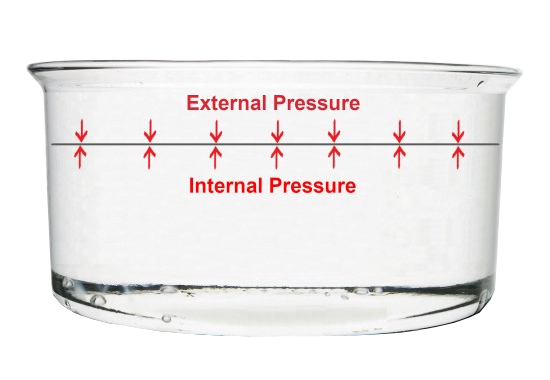
If these pressure were different at all points on the surface of the water, it would not remain flat but undulating with dents and lumps.
Therefore, the pressures are uniform across all points, so the water surface remains flat and horizontal.
As long as the internal pressure of the water remains less than or equal to the external pressure (that is, atmospheric pressure), all the water will stay in the saucepan.
Now, if we place the pan on a burner, the water will receive energy in the form of heat.
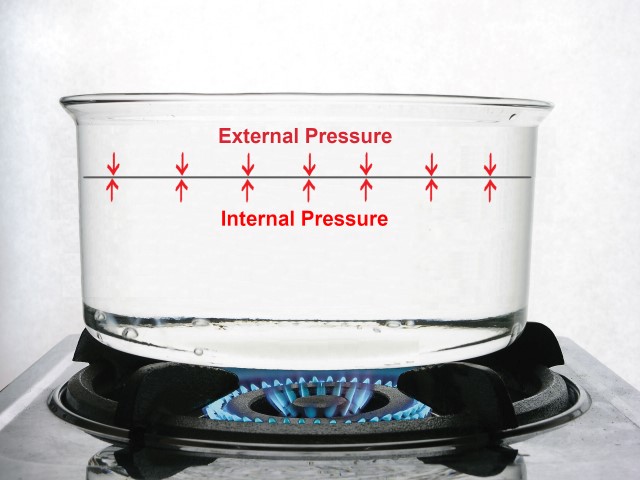
This energy causes an increase in the internal pressure of the water, just as the air you blow into the balloon causes the membrane to stretch.
Gradually, as the burner heats the water, the internal pressure of each droplet increases more and more. However, the external pressure (atmospheric pressure) doesn’t change.
As soon as the internal pressure exceeds the external pressure, the external pressure will not be able to keep the water in the saucepan.
At this point, the water molecules break apart from each other and become gas molecules lighter than air, leaving the saucepan.
It is why large bubbles of water vapour (steam) burst out from the surface, causing a drop in the level of liquid in the pan.
Although we continue to heat the saucepan, the temperature is no longer rising. It is because all the heat from the stove only serves to make the water molecules escape and causes the change of state: This is the vaporisation step!

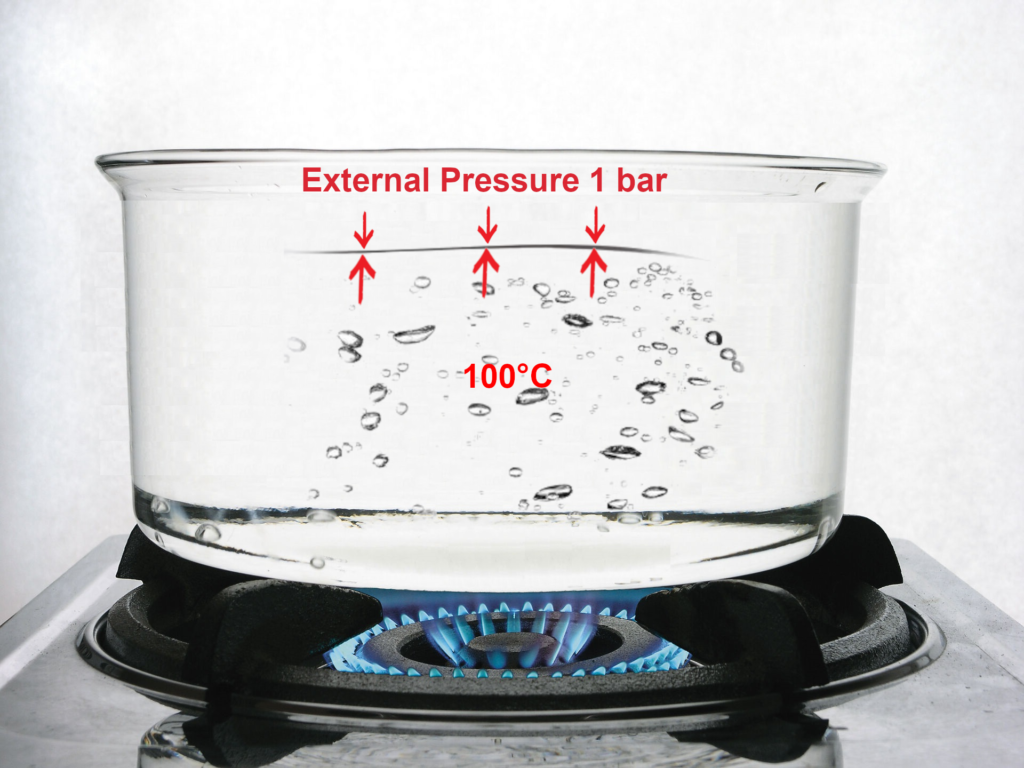
At sea level, atmospheric pressure, and hence the external pressure, is 1 bar.
Therefore, we need to raise the water temperature to 100°C so that the internal pressure can overcome the atmospheric pressure and so boiling can start to occur.
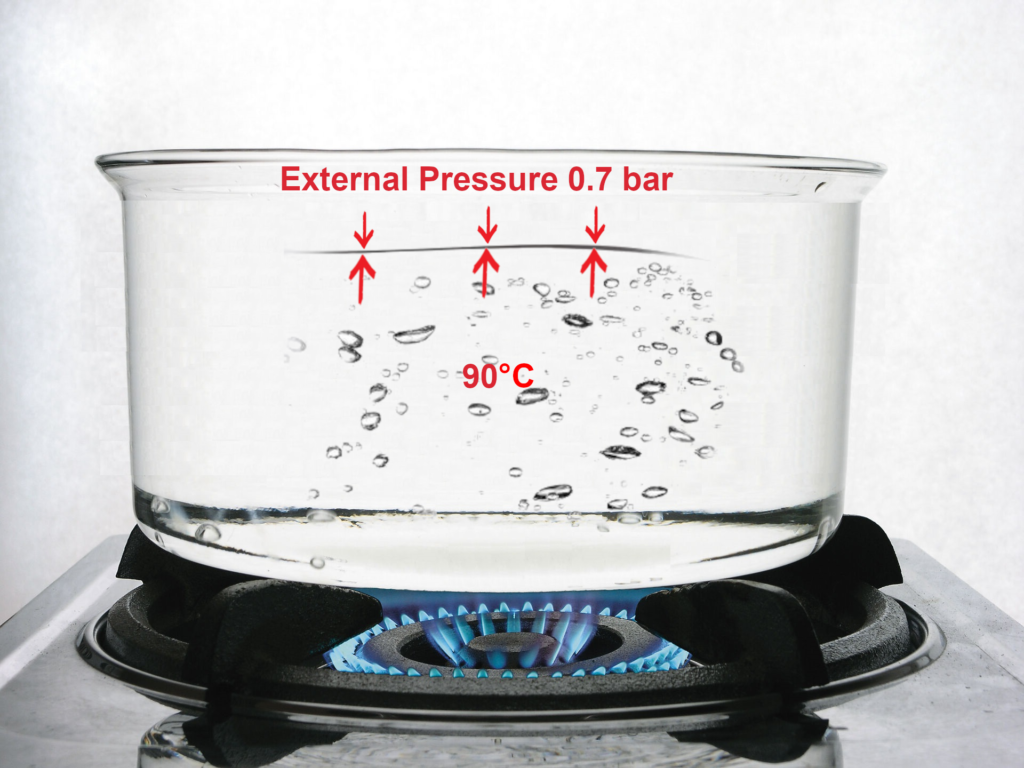
In the mountains, as the altitude increases, the thickness of the atmosphere decreases. Therefore, it causes a reduction in atmospheric pressure and a reduction of external pressure.
As the external pressure decreases, the internal pressure needed to allow the water molecules to escape from each other also becomes smaller. As a result, water will start to boil at less than 100°C.
What do you think the boiling point of the water would be if the external pressure increased: would ii’ stay at 100°C, would it increase, or does it decrease?
Just think about the operating principles of a pressure cooker.
Everyone knows a pressure cooker: a special saucepan with a sealable cover. When you put water in the pressure cooker and seal the lid, the water at the bottom and air at atmospheric pressure remains trapped inside the pressure cooker.
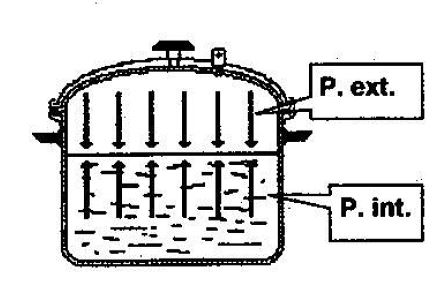
As you heat the pressure cooker, the water’s temperature rises simultaneously, and the internal pressure of the water increases. So whilst the water temperature remains at less than 100°C, the external pressure (inside the pressure cooker) remains sufficient to keep the water in liquid form at the bottom.
Once it reaches 100°C, only a tiny amount of extra energy is needed to allow the first water molecules to escape from each other. These vapour molecules (water in its gaseous form) remain trapped inside the pressure cooker.
There is, therefore, more and more gas inside the cover. It produces the same effect as when you blow air into the balloon (that is, you add some gas to the interior of the balloon)—the pressure inside the pressure cooker increases.
Since the external pressure has increased, boiling is prevented, despite the water being at 100°C.
However, as it continues to heat the pressure cooker, the internal forces of the water molecules continue to increase. As soon as they become greater than the external forces, boiling occurs at an excess of 100°C.
The extra water vapour that this releases also remains trapped inside the cover of the pressure cooker, so the external forces increase further.
Boiling is again prevented, despite the water being at a temperature greater than 100°C, and so on. As this process continues, the water temperature gradually rises as the external pressure increases.
If the pressure in the pressure cooker increases indefinitely, it would be dangerous with the risk of explosion. So the manufacturers fit a pressure-reducing valve in the pressure cooker cover to prevent this danger. However, the valve opens and releases steam if the pressure becomes too great. When this happens, the pressure releases to keep internal pressure safe.
This valve opens to release water vapour as soon as the pressure inside the pressure cooker reaches about 2 bar. At this pressure, the balance with the internal forces of the water is at about 120°C.
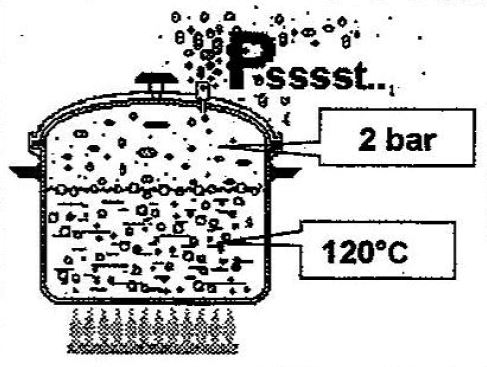
This way, the valve maintains 2 bar in the pressure cooker, and the water temperature stays at about 120°C. Of course, dishes cook much more quickly at 120°C than in a traditional saucepan at atmospheric pressure, where the water is only at 100°C. In summary, the boiling temperature of water changes in line with pressure. They increase or decrease together.
Every time the pressure changes, the boiling temperature also changes. So, in effect, every pressure has a corresponding boiling point. It is what is known as The Pressure-Temperature Relationship.
The refrigeration industry doesn’t call this boiling, but rather vaporisation or evaporation.
The diagram below shows the pressure-temperature relationship for pure water. It shows the evaporation temperature of the water as a function of the external pressure.
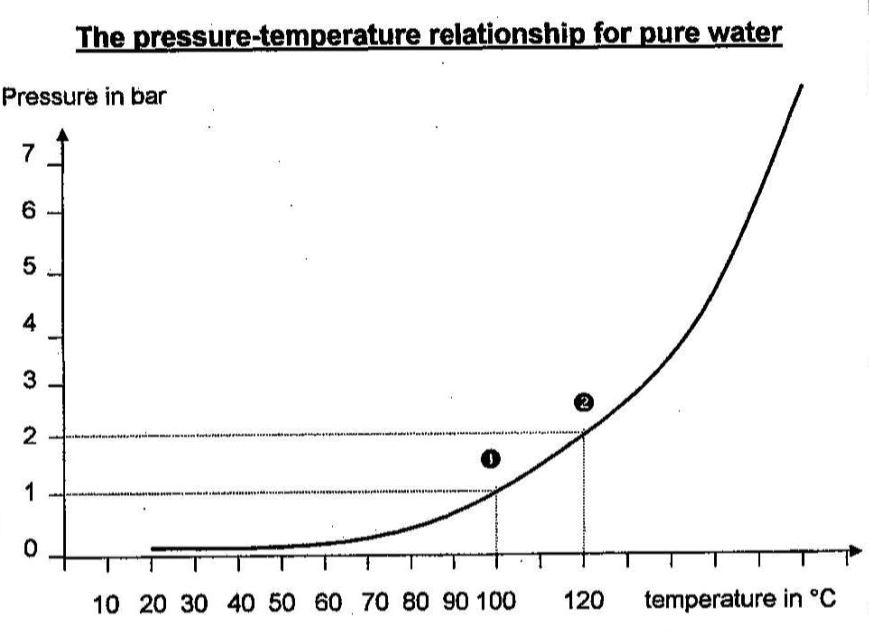
In this diagram, we can see that at 1 bar. (that is, atmospheric pressure), water boils at 100°C (point 1 ). We can also see that at 2 bar, water boils at 120°C (point e 2), and so on.
This diagram confirms that if the pressure increases, the evaporation temperature also increases. Similarly, if the pressure falls, the evaporation temperature decreases likewise. Evaporation temperatures and pressures always vary in the same direction. They increase and decrease together. They are linked.
It is essential to note.
- There can only be heat transfer between two bodies if those two are at different temperatures.
- Heat always flows from the hotter body to the colder body.
- The change from the liquid state to the vapour state is called evaporation.
- To evaporate, it must absorb heat.
- During the evaporation of a liquid, the temperature remains constant. The heat absorbed by the liquid during evaporation is called the latent heat of evaporation.
- Evaporation temperatures and pressure always vary in the same direction. They increase and decrease together. They are linked.
The processes of evaporation are essential in the operation of a refrigeration system.
Back to Basic!
Related
Read more: Fan wall
Read more: How to verify the percentage of outside air in an enclosure
Read more: BCA Part J5 Air-conditioning system control
Read more: Microbial Induced Corrosion (MIC) in Pipes
Read more: Is your kitchen exhaust system a fire hazard
Read more: What is coanda effect